Practical Tricks to Start a Fire in Stoves or Nature
The Simplified Science of Fire Initiation and Propagation
Starting a fire in a wood stove or in wild nature is not as easy as movies make it look like! But once you learn the process and some practical tricks, it could be a useful skill for camping, when stranded in the cold wild, or being self-reliant and enjoying the heat from your own rustic woodstove (and your own dead tree logs instead of depending on fossil fuels).
In this article, I share what works for us and some of the science of how fire works.
Stages of Fire
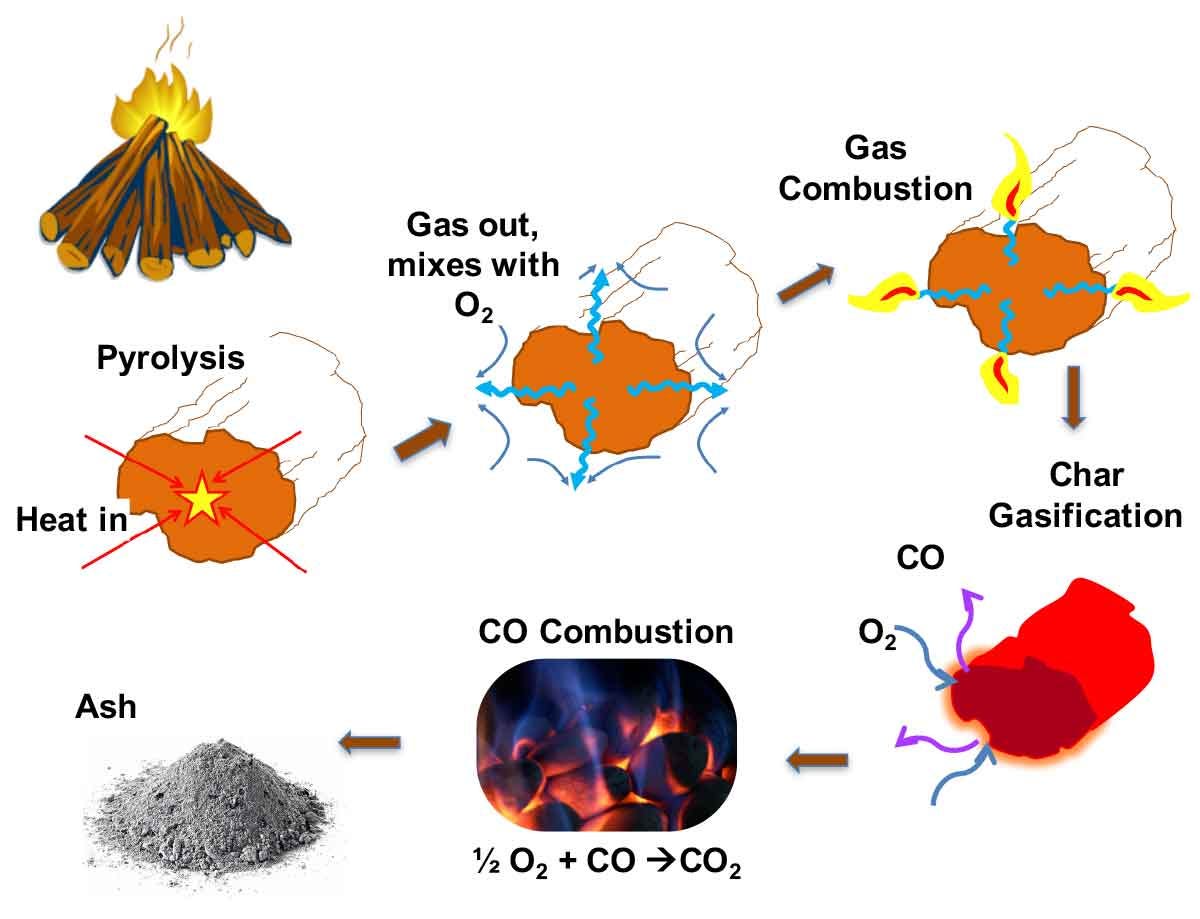
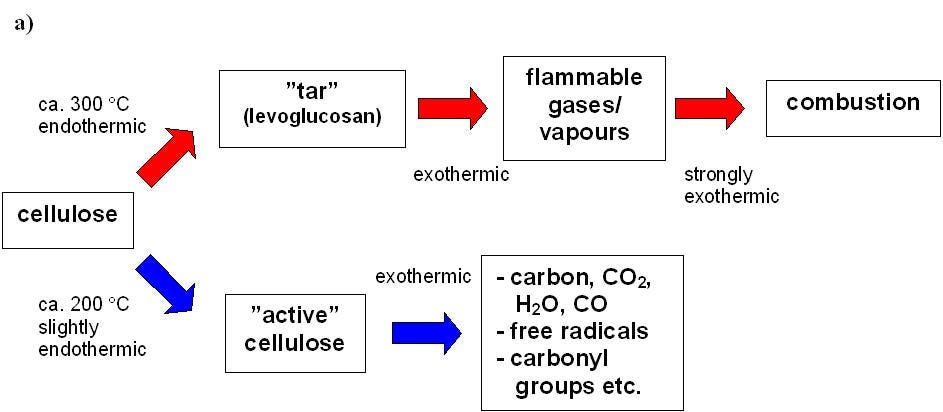
Evaporation and Pyrolysis: As long as we have moisture in the wood, we cannot reach the flash point temperatures. Water has a higher specific heat (energy needed to raise the temperature of a unit mass) than dry wood, and heating up and evaporating water consumes a lot of heat. Wood burns best at a moisture content of less than 20 percent. Once water is mostly evaporated (for which we need to raise the temperature of all wood surfaces to around 100 °C or 212 °F), supplying further heat will help with the process of pyrolysis (i.e. thermal decomposition) on the wood surfaces exposed to the flame or heat. Depending on environmental conditions (such as temperature, oxygen concentration, moisture and type of wood), the pyrolysis of wood can proceed mainly on two pathways. The tar forming pathway (temperatures around 300 °C or 572 °F), is related to the “normal” burning of wood. It produces tar that decomposes easily into burning gases under the influence of heat. Thermal decomposition can take place also through char forming pathway, which produces carbon dioxide and water, and a cellulose-based char.
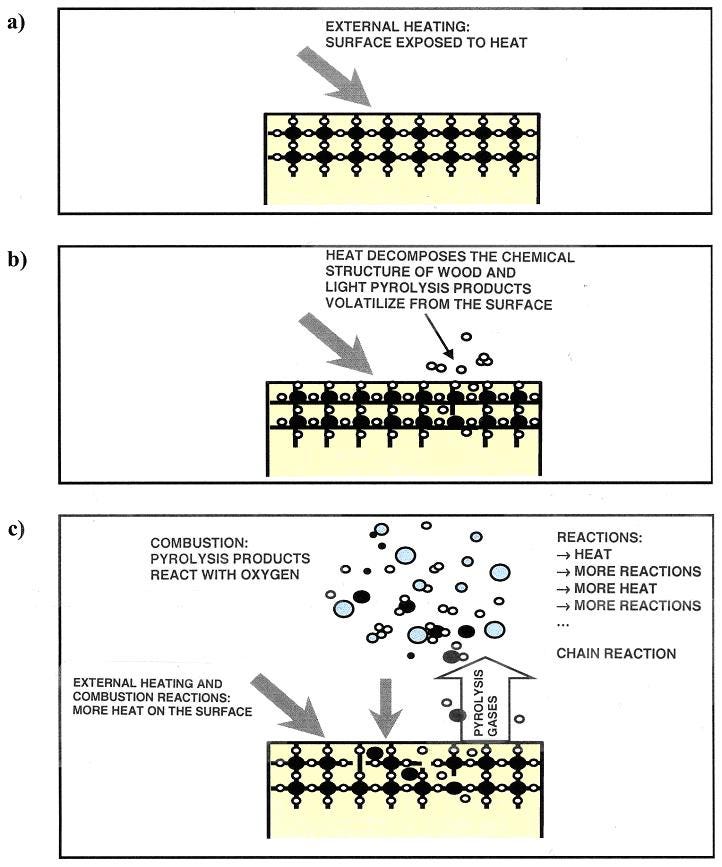
Ignition and Combustion: Fire is generated when oxygen reacts with gases released from pyrolysis (except for the “glowing” combustion of charred wood where oxygen reacts directly with carbon). Pyrolysis and combustion release a large amount of heat that induces further pyrolysis and combustion reactions, making the combustion a “chain” or “auto-catalytic” reaction that can feed itself with enough energy to sustain (without additional energy input). Chemical engineers use these terms to refer to reactions that feed back energy and material to be self sustaining. An extreme example of a “chain” auto catalytic reaction is explosion (extremely fast combustion). The fire will be self-sustaining if the rate of heat generated by combustion (which depends on rate of oxygen flow, surface area of fire, rate of cooling and air flow, etc.) can bring enough wood beyond the point of further pyrolysis and combustion. Factors such as density, specific heat, surface area and thermal conductivity of the fuel (wood) and speed of ventilation are important determinants of how self-sustaining and fast-spreading the flames are.
The hottest part of the flame is the base, so this typically burns with a different color to the outer edges or the rest of the flame body. Blue flames are the hottest (around 2000°C or higher), followed by white. After that, yellow, orange and red (525°C to 1000°C) are the common colors you’ll see in most fires. A propane torch or flame burns at 1900°C (hence often blue), a candle at around 1000°C and a household wood fire at around 600°C (1100 °F, orange and red). A schematic picture of pyrolysis and combustion of wood follows:
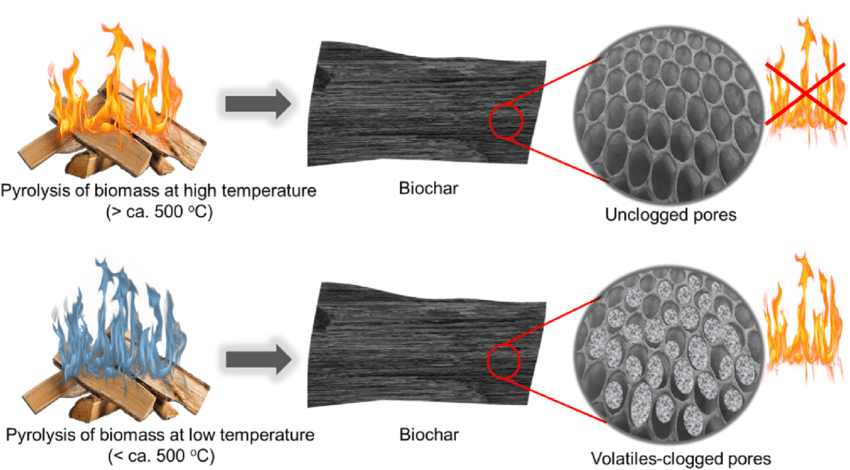
Charring: When a wood product burns at a constant rate of heat release per unit area, the boundary between the pyrolysed material and the intact wood, i.e. the pyrolysis front, proceeds to the wood in depth direction. A typical value of the charring rate of wood is approximately 0.5 - 1 mm/min. In fire retardant wood, a porous, carbon-rich layer is formed on the surface of wood and the reactions would often proceed so that cellulose decomposes to char and water: (C6H10O5)n → n(6 C + 5 H2O). This is why sometimes a medium-temperature pyrolysis stage that avoids fast conversion to char may actually produce a hotter longer-lasting fire.
Practical Tricks to Start A Fire
Compartment fire development can be described as being comprised of four stages: incipient (evaporation and pyrolysis), growth (ignition), fully developed (combustion and chain reaction) and decay (charring).
The success of starting a fire with ease is to ensure that the initial ignition generates enough heat that (1) Evaporates moisture, (2) Maintain pyrolysis of the volatiles, (3) Carries on further pyrolysis and combustion. This is why using “kindling” is so important. Kindling is any thin and dry piece of wood or paper that ignites much easier (because it has a lower thermal mass and higher surface area exposed to oxygen) than thick logs. A thermally thin layer (less than 10 mm) ignites more quickly than a thermally thick material because when heated on one side, the thin material’s opposite side heats up to temperatures close to those of the exposed side by the time to ignition. Lighter (lower density) wood species and paper make better kindling than heavier wood. External factors having an influence on the ease of ignition are the surface area of kindling exposed to ventilation and oxygen flow, and entrapment of heat generated by the ignition so it can be efficiently captured for pyrolysis and ignition of the adjacent fuel (larger pieces of wood).
My favorite method is to ….
Science today is mostly a profitable and sponsored business. Be wise. Don’t sell your attention to “attention merchants.” Get your information by supporting “independent” (non-corporate) God-fearing scientists who share useful insight and uncensored science. I am a scientist guided just by my God-given conscience, not by mobs, politics, profits or corporations.
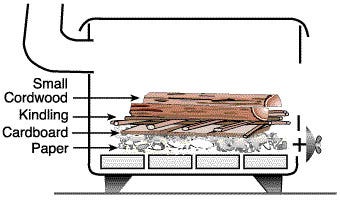
.. bunch some newspapers, and lay them together with some thicker kindling (like cardboard, dry twigs or thin strips of bark or wood1) like a pyramid with adequate internal air flow close to the air inlet of the stove. The idea is the most ignitable flammable material release heat to support burning of the slightly more stubborn fuel, which will in turn burn and support progressively larger, denser pieces. This will continue until we have enough heat released to sustain the fire for thermally thick mass. For cold days or when we don’t have enough twigs or kindling, I use small pieces of a starter material (compressed fuel like the Timberlite brand). Diligent wood-stove operators have all the necessary fire-starting materials readily available at the stove site with some matches (or flintstones, or fire starter stones in nature). Open the stove vent as far as it will go, and light the fire (Figure 1). If there is not enough oxygen for the burning surface during the ignition phase, as seen in the graph shown above, we will face a temperature drop and difficulty in maintaining a steady propagating flame.
As seen in the picture here, I surround my kindling fluff (newspapers, twigs, starters) with a bridge-like structure using larger thicker pieces of dry seasoned wood 2) that not only captures the ignition heat but also provides an air/oxygen flow conduit. You want to adjust the air inlet vents to make sure the air flow provides enough oxygen to the fledgling fire but does not cool it too fast, waste too much heat through the chimney, lower the ignition temperature and delays arriving at (self-sustaining) self-propagation (chain reaction) temperature of 300 °C (572 °F).
Once the combustion (chain reaction) is maintained, we want to build up a hot bed of coals and ensure we have enough flammable surface area exposed to the hot coal or flame, and enough oxygen flow but not too much cooling and heat removal (ventilation through chimney) so we may start closing the air inlet valve as we approach our desired fire temperature. You can use inexpensive temperature gauges or guns to measure the stove’s top surface temperature (which is lower than the internal combustion chamber but gives you an indicator to adjust air flow to the stove). A nice wood fire will burn at around 1000 °F (internally, but perhaps closer to 600 °F on the stovetop or walls). For a wood fire to burn efficiently and cleanly, it must burn hot most of the time.
Additional Notes and Tricks
Cold, Windy Days: Draw refers to the movement or flow of air from the inside of the house, through the vent and fire box, and up the chimney. Draw is created by physical factors such as differences in pressure or temperature between the fire box and the top of the chimney. On cold or windy days, and when air inlet vents are open, adequate upward draft or draw ensures oxygen flow is not the rate-limiting step in our fire. Experienced wood-stove operators do not like to start a fire when outside temperatures (chimney outlet) are higher than 10 °C (50 °F). On the other hand, too much draw (on very cold or windy days) will waste a lot of heat through the chimney and cools down our reaction media. We may even have a negative (down draft) pressure and draw. To start a fire when your wood stove has negative pressure (very cold days), first ensure you have plenty of dry materials. Wad five or six sheets of newspaper, and place the wad below the stovepipe leading into the fire box. Ignite it. The paper will burn rapidly, warming the chimney. This rapid increase in temperature creates a positive draw. Quickly add more paper and fine kindling. This additional fuel heats the chimney more and eventually establishes a favorable situation for a continued fire.
The Hierarchy of Surface and Mass: In college, I found combustion engineering an extremely complex subject because it involves mass, heat and momentum (convective) transfer. But the overall idea can be simplified as follows: The rate of heat available for further continuation of fire is determined by the surface areas being burned (the larger, the more heat released) and the thermal mass (inertia) of the remaining fuel (the less, the better). So it’s a great idea to start from long thin pieces with high surface area but low mass (like twigs or newspapers) that are dry (no waste of energy in evaporation of moisture). The heat released would be enough (if captured in a compartment like a firebox or the bridge structure I use) to support a temperature rise (to the pyrolysis point) for the next level of fuel with an intermediate surface area/thermal mass ratio (lower than the kindling but higher than the large fire logs). By creating this type of hierarchy (of surface area to mass), we ensure that faster (but shorter lasting, low mass) fuel sustains the chain reaction of the slower (but hotter and longer lasting) fuel. In every step of this process, we need to play with the oxygen flow and venting rates to control the reaction rates and cooling rates.
Safety: You must observe all fire safety guidelines and ensure there is no build up of creosote and the risk of fire inside the chimney. Always have fire extinguishers handy and close to the stove.
Avoid pine because it generates a lot of resinous tar and creosote
We use ash trees that already fell in our land or in neighbor’s, and also have access to denser species such as oak too. Never use coated, painted, pressure-treated, laminated or engineered wood, ocean driftwood, plywood, particle board, or any wood with glue on or in it. Also avoid wet, rotted, diseased, or moldy wood. Use seasoned (low tar, weathered and aged) dry wood species like ash, oak and maple.