Facts and Myths About Carbon Dating
Useful Information for Mel Gibson, Joe Rogan, Evolutionists and Creationists
Many devout Christians, based on biblical verses in Genesis, believe humans have been on the planet for about 8,000-10,000 years, starting with Adam and Eve, whom were created by God as first humans and were not genetic descendants of primates like monkeys. Contrary to this “creationist” view, evolutionists, relying on Darwin’s work, believe current humans (Homo sapiens), a species from the genus Homo (of great apes), have been around for at least 200,000 years and preceded by other Homo species like Homo erectus which appeared about 2 million years ago. Evolutionists base much of their arguments of on “carbon dating” (or radiocarbon dating) and other dating techniques of human fossils and skulls from early hominids.
Recently, actor Mel Gibson, who is a creationist, was asked by Joe Rogan about his views about carbon dating, which is used to confirm evolutionists’ claims about the age of the planet and our species. Gibson said he questions “carbon dating” but Rogan says “Carbon dating seems rock solid. The science behind it is pretty legit!.” (minute marker 6 of following video). Gibson could not explain why he doesn’t believe in carbon dating. So I decided to write this.
Carbon dating is useful in some cases but has several flaws. Here is the simplified science of carbon dating and its shortcomings. Other dating techniques have their own flaws, which are beyond the scope of this article. I am listing these flaws with “carbon dating” not to defend or reject creationism, but to show the little-known but serious limitations of this modern scientific tool and technique. I have spent many years in science labs and need to share this with the public who believe characterization techniques are fool proof. This article is not about my views about evolution. I can discuss that separately.
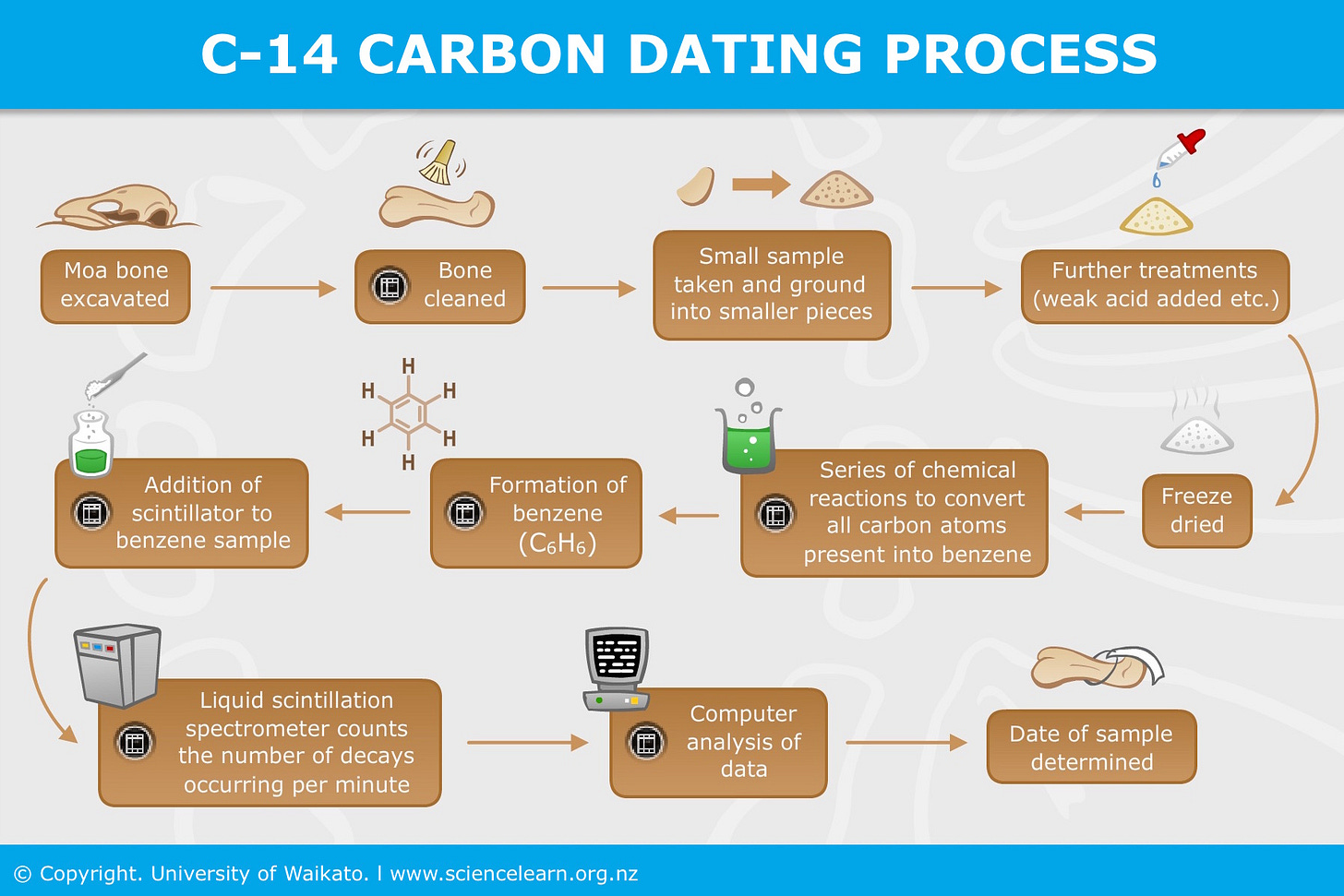
Carbon Dating
If you look at the periodic table, nitrogen, the most abundant element in the “atmosphere,” is right after carbon, the most abundant element in organic matter (like plants and animals). A small fraction of nitrogen in the atmosphere is converted to an isomer of Carbon (called Carbon 141), and absorbed as carbon dioxide by plants and then other living organisms. As long as organisms are alive, the ratio of carbon 14 to regular carbon (C12) is “assumed” to be constant, but as soon as they die, they stop breathing and metabolizing carbon 14 so the ratio drops over time. For dating an organic sample, researchers measure both radioactive carbon (called C14) and the commonest form of non-radioactive carbon (C12). They calculate the ratio between the two amounts and compare this with the known ratio in living organisms. This tells them how much C14 has decayed. Since, the rate at which C14 decays is already known (it decays to half its amount every 5,730 years), having worked out how much has decayed in the sample, researchers can calculate the time it would have taken for that amount of decay – in effect, the age of the sample since it died.
Serious Flaws
The technique is not a robust scientific method because it measures very small amounts of an element (C14) that can easily be contaminated, in an elaborately processed and altered sample (see steps above). Radiocarbon samples are easily contaminated, so to provide accurate dates, they must be clean and well-preserved. But old samples have often dirt and microbial matter that are carbon-based. Because there are so few atoms of C14 to count, even a little extra carbon from contamination will throw off the results significantly. One well-known example of carbon dating being off by thousands of years is when scientists initially dated the remains found in the Darra-i-Kur cave in Afghanistan as what they call Paleolithic humans from 30,000 years ago (disproving the Christian belief in humankind’s less than 10,000 years history), but later analysis of skull fragments revealed they were much younger, from only 4500-5000 years ago! A discrepancy of 25000 years due to issues with the carbon sample quality.
Keith and Anderson radiocarbon-dated modern mollusk shells from freshwater rivers and found errors on the order of several thousand years! They report: “Errors of shell radiocarbon dates may be as large as several thousand years for river shells.. owing mainly to incorporation of inactive (carbon-14-deficient) carbon from humus, probably through the food web, as well as by the pathway of carbon dioxide from humus decay. The resultant effect, in addition to the variable contributions of atmospheric carbon dioxide, fermentative carbon dioxide from bottom muds, and, locally, of carbonate carbon from dissolving limestones, makes the initial carbon-14-activity of ancient fresh-water shell indeterminate...”
The technique does not account for unknown but certain fluctuations in atmospheric carbon-14 levels over time2 caused by solar winds and other factors. The proponents of the technique admit it is unreliable for dating items after 1950 because of the significant man-made changes in the atmosphere such as increase in atmospheric carbon-14 caused by nuclear weapons testing. I also believe carbon dating cannot reliably work with samples older than 11,400 years old or so given the half life of carbon 14 which is 5700 years. In samples older than 11,400 years old, C14 would decay to one fourth of its already small original amount and not easy to measure with accuracy. Another problem with the changing nature of the C14 in the atmosphere is the circular nature of arguments about climate change that use C14 dating.
Fossil bones incorporate new radiocarbon by means of recrystallization and, in some cases, bacterial activity and uranium decay. Because of this, bone mineral – fossil or otherwise – is a material that cannot yield an accurate radiocarbon date.
Diet, a major source of carbon intake and deposit in animals and humans, changes drastically over time and history affecting the amount of carbon-14 in tissues, potentially leading to inaccurate dating.
Like most ither analytical techniques, C14 dating relies heavily on calibration curves. Researchers at Cornell University, find differences, on average, of 60% between the 95.4% probability ranges determined from calibration curves versus those approximately allowing for the observed offset pattern. Such differences affect, and even potentially undermine, several current archaeological and historical positions and controversies.
Sources:
https://www.pnas.org/doi/10.1073/pnas.1719420115
https://www.sciencedirect.com/science/article/abs/pii/S0047248417301136
https://pubmed.ncbi.nlm.nih.gov/17781758/
14 is the atomic mass of Carbon 14. Regular carbon has an atomic mass close to 12, which is the average total number of protons and neutrons in their atom’s nucleus.
For example, cosmic radiation is now forming C-14 in the atmosphere about 134% faster than it is decaying, which shows the variable nature of C-14.





Fascinating. I always wondered about carbon dating. I’m a creationist myself. I’ve always thought that our history and limited, oftentimes contradictory, records were manipulated, hidden, and/or fragmented. Carbon dating has been largely accepted as settled “science “. Which, there truly seems to be no such thing.